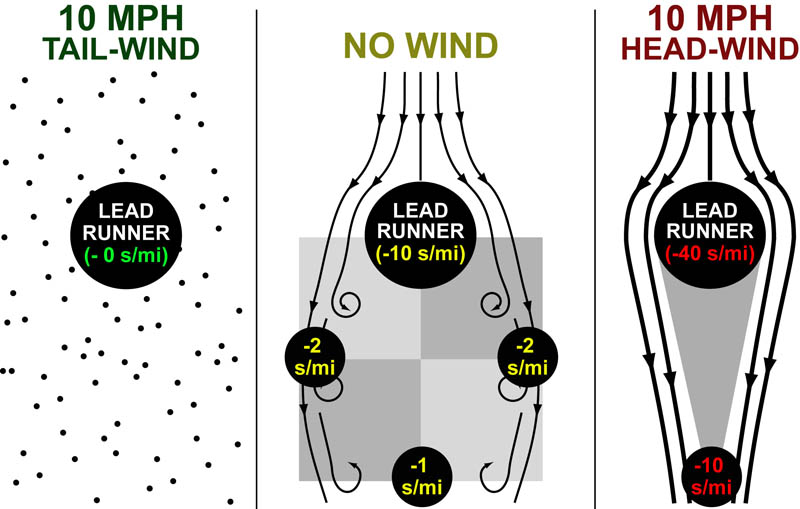
Recently, while running a workout on a windy track, I started wondering about the science of air-resistance and drafting. More specifically, I wondered what difference was between 10 mph tail-wind and the 10mph head-wind I encountered on opposite sides of the track (see figure above). When I returned home, I discovered some classical papers that answered this exact question in an experimental and theoretical way (summarized in the Figure above).1-4
About Us
Practically Science was started by two Yale PhD students in 2012. Its goal is to make single-sheet summaries of common interdisciplinary methods, ideas, etc. Continue Reading →Please follow & like us :)
Blogroll
Site Map
-
Recent Posts