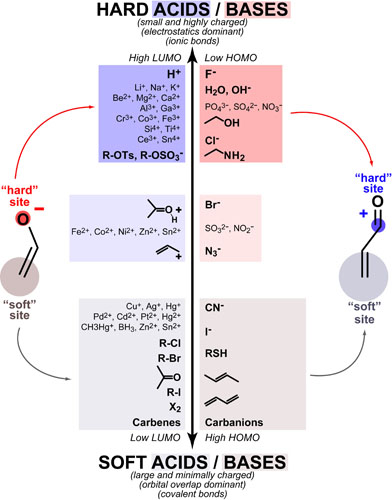
Hard-Soft Acid-Base(HSAB) theory one of the most useful rules of thumb for explaining and predicting chemical reactivity trends. Hard molecules tend to be small/non-polarizable and charged while soft molecules tend to be large/polarizable and uncharged. Both acids/electrophiles and bases/nucleophiles can be hard and soft and the defining reactivity rule of HSAB theory is:
- hard acids prefer to react with hard bases forming ionic bonds
- soft acids prefer to react with soft bases forming covalent bonds
HSAB REGIOSELECTIVITY:
These rules are especially useful for rationalizing regioselectivity in ambident molecules (molecules that have more than one reactive site) such as enols and enones (see figure above):
- hard acids react with the oxygen of the enolate
- soft acids react with the carbon of the enolate
- hard bases react with the carbonyl carbon of the enone
- soft bases react with the β-carbon of the enone
KLOPMAN EQUATION EXPLAINS HSAB THEORY:
Finally, though these rules were originally derived empirically, there is quantum mechanical explanation for these trends in the Klopman equation:
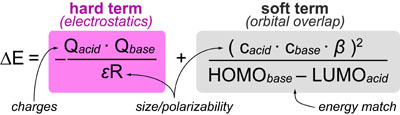
This equation shows how chemical reactivity is defined by two terms: (1) an electrostatics (ionic) term and (2) orbital (covalent) term. In general:
- hard/hard interactions maximize the electrostatic term (i.e. highly charged molecules are very attracted to each other)
- soft/soft interaction maximize the orbital overlap term (i.e. molecules with similar energy HOMO’s and LUMO’s most easily make covalent bonds)
REFERENCES:
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533-3539.
- Pearson, R.G.; Songstad,J. Application of the Principle of Hard and Soft Acids and Bases to Organic Chemistry J. Am. Chem. Soc. 1966, 89, 1827-1836.
- Klopman, G. Chemical Reactivity and the Concept of Charge- and Frontier-Controlled Reactions. J. Am. Chem. Soc. 1968, 90, 223-234.
- Pearson, R.G. Hard and Soft Acids and Bases, HSAB, Part II. J. Chem. Educ. 1968, 45, 643-648.
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions, Wiley-Interscience, 2004
- McQuarrie, D.A.; Simon, J.D. Physical Chemistry: A Molecular Approach. University Science Books, 1997
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, Part A: Structure and Mechanisms. Springer 2008

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.