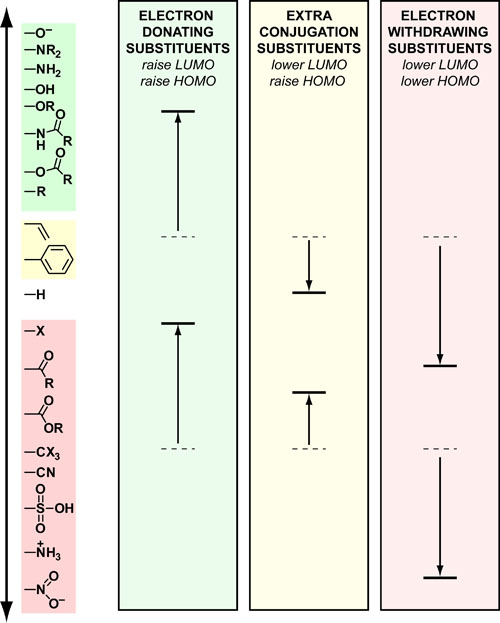
Often, if you are trying to design a molecule which has function (e.g. catalysts, fluorophores, switches, etc.) you have to tweak the electronics of that molecule. Generally, the most important electronic energy levels are the HOMO’s and LUMO’s which can donate and accept electrons, respectively. The figure above summarizes the substituents that are most used to raise the energy (electron donating groups), lower the energy(electron withdrawing groups) or do both (extra conjugation groups).
- Electron donating groups (EDGs) destablize pre-existing energy levels by increasing electron-electron repulson.
- Electron withdrawing groups(EWGs) stabilize pre-exisiting energy levels by increasing electron delocalization.
- Extra conjugation groups are a bit more complicated but can be understood in terms of linear frontier molecular orbital theory
REFERENCES:
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions, Wiley-Interscience, 2004
- McQuarrie, D.A.; Simon, J.D. Physical Chemistry: A Molecular Approach. University Science Books, 1997
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, Part A: Structure and Mechanisms. Springer 2008

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.