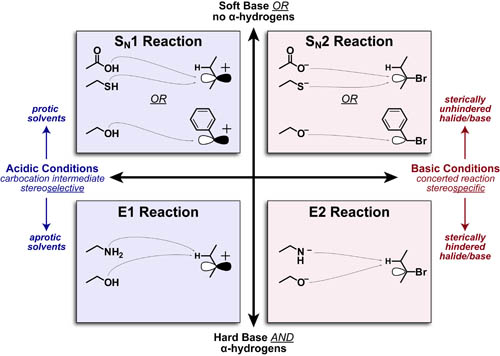
Substitution and elimination reactions (between Lewis-bases and alkyl-halides) are some of the first reactions taught in organic chemistry. The figure above, organizes the main factors that distinquish: SN1, SN2, E1 or E2 mechanisms into a single, 4-quadrant spectrum. We describe the heirarchy of these factors in more detail below.
- Acidic/Cationic conditions or Basic/Anionic conditions distinquich “1” vs “2” mechanism, respectively.
- Elimination requires alpha hydrogens and predominates with 2-3° alkyl-halides.
- Elimination predominates with harder bases (due to stronger electrostatic interactions) while Substitution predominates with softer/nucleophilic bases(due to better orbital overlap). See post on Hard-Soft Acid-Base Theory for more details.
In addition the most general rules outlined above:
- Under Acidic conditions: Substitution is favored with protic solvents (due to stabilization of the carbocation intermediate)
- Under Basic condition: Elimination is favored with higher substitution of either halide or base
REFERENCES:
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions, Wiley-Interscience, 2004
- Grossman, R.B. The Art of Writing Reasonable Reaction Mechanisms, Springer 1999
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, Part A: Structure and Mechanisms. Springer 2008

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.