Most plant pigments seem to have evolved as “sunscreens” for the photosynthetic machinery of the plant wherein they filter out high energy (potentially damaging) light that cannot be used for photosynthesis. Chemically there are only a few classes of these “sunscreens” whose light absorbing properties lead to rich array of colors that we observe throughout the plant kingdom(see figure above). Below we discuss each class in terms of common observations (e.g. autumn leaves) and cooking considerations.
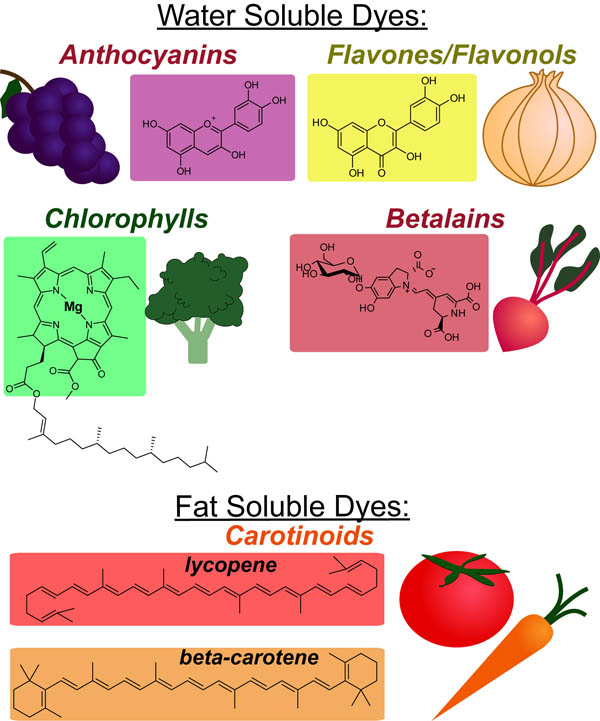
Water Soluble Dyes:
Most water soluble dyes (Anthoxanthins, Flanvonols, Flavones, Betalains) are stored with starch (and typically glycosylated) in the storage vacuoles of plant cells. Cooking damages these vacuoles causing these dyes to leak and be lost (diluted out) during boiling. Typically, steaming or rapid boiling can prevent this mechanism color loss. In addition these compounds exhibit differential acid/stability. Specific recommendations are listed below:
ANTHOCYANINS:
Source: most fruits and vegatables that are colored red, purple or blue
Acid/Base Sensitivity: red in acid, purple/blue at neutral, green/yellow in base
Cooking Recommendation: Add acid (lemon juice, vinegar, etc.) to counteract basic tap-water
FLAVONOLS/FLAVONES:
Source: potatoes, onions, cauliflower
Acid/Base Sensitivity: white in acid, , yellow in base
BETALAINS:
Source: beets, chard, mushrooms
Acid/Base Sensitivity: relatively stable
Cooking Recommendation: not metabolized so color urine intensely
CHLOROPHYLLS:
Source: green plants (leaves)
Acid/Base Sensitivity: Acid dulls color
Cooking Recommendations:
- Dilution: Chlorophyll’s hydrophilic tail keeps anchored in chloroplast membrane (preventing dilution) but can be removed by acid, base or heat. Short boiling in tap water is preferred to prevent dilution (by preventing leakage).
- Color: steaming exposes chlorophyll to plant-cell acids which will dull color. Short boiling in tap water (slightly basic) is preferred to retain color (by neutralizing plant cell acid).
Fat Soluble Dyes:
The carotenoids are fat/membrane soluble and concentrated in the membranes of chloroplasts where they absorb UV/blue radiation to protect the chlorophyll. A few more details are given below.
CAROTENOIDS::
Source: most fruits and vegetables that are yellow or orange
Acid/Base Sensitivity: stable to acid/base
Cooking Recommendation: stable to cooking as stay in membrane of plant cells (not soluble in water)
ENZYMATIC BROWNING:
The “browning” one observes when cutting/crushing fruits and vegetables is a chemical defense mechanism of plant cells against damage/invaders. This “browning” is a chemical polymerization that requires phenols (stored in vacuoles) enzymes (stored in the cytoplasm) and oxygen (excluded from inside of the cell). When plant tissue is damaged these three components are mixed at the site of damage causing polymerization and browning. Browning can be prevented by: (1) excluding oxygen (vacuum packing); (2) denaturing the cytoplasmic enzymes by rapid boiling (blanching) or adding acid (e.g. lime juice prevents guacamole browning); or (3) rapid freezing to stop the chemical reaction.
REFERENCES:
- McGee, Harold. 1984. On Food and Cooking. New York, NY. Scribner pp. 272-274,389-400.
- Potter, Jeff. 2010. Cooking for Geeks: Real Science, Great Hacks, and Good Food. O’Reilly Media
- Delgado-Vargas, F.’ Jimenez, A. R.; Paredes-Lopez, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains — Characteristics, Biosynthesis, Processing, and Stability Critical Reviews in Food Science and Nutrition, 2010, 40, 173-289.

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.