There are many drugs that act as agonists ligands (L) which means they “turn on” their target receptor (RL) so that it induces its normal down-stream signalling. Examples of such drugs include: growth hormones, insulin, steroids and G-protein coupled Recetor(GCPR) ligands such as morphine (opiods), neurotransmitters and scent/aroma compounds. In general you can improve the potency (EC50) of these drugs by improve their binding dissociation constant (Kd) for their receptor (for more detail see post on the Hill Equation).
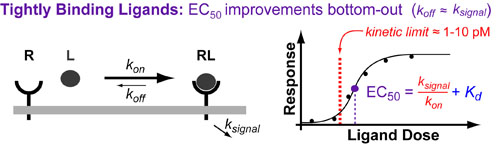
The Kd is equal to the ratio of the complex dissassembly rate contant (koff) over the complex assembly rate constant (kon). In general, kon is a diffusion-controlled constant defined by how quickly the receptor and ligand find each other (and collide with the right geometry). As such, in general, you can only improving the potency of a drug/ligand ([EC50), by reducing reducing its koff. This makes sense because: the “tighter” your drug binds the less it will “fall off” the receptor.
Unfortunately, there is a limit to how far engineering the koff can improve your ligand potency (EC50). Once your ligand, binds so tightly that koff is less than the rate constants for downstream signalling(ksignal) (or receptor recycling/endocytosis (kendo) the EC50 becomes as constant which is approximately equal to ksigalling / kon. This “kinetic-limit” is a basic thermodynamic limit which has been defined to occur around 1-10 pM through extensive theoretical and experimental work on the drugs described above (see references below)
REFERENCES:
- Foote, J.; Eisen, H.N.; Kinetic and affinity limits on antibodies produced during immune responses. PNAS, 1995, 92, 1254-1256.
- Lauffenburger, D.A. Receptors: Models for Binding, Trafficking and Signalling, Oxford University Press 1993.
- Batista, F. D.; Neuberger, M. S. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 1998, 8, 751-759.
- Pearce Jr, K.; Cunningham, B.; Fuh, G.; Teeri, T. Growth hormone binding affinity for its receptor surpasses the requirements for cellular activity Biochemistry 1999. 38. 81-89.
- Matthews, D. J.; Topping, R. S.; Cass, R. T.; Giebel, L. B. A sequential dimerization mechanism for erythropoietin receptor activation. PNAS 1996, 93, 9471.
- Radhakrishnan, M.; Tidor, B. Cellular level models as tools for cytokine design. Biotechnology Progress. 2010, 26, 919-937.
- Kalie, E.; Jaitin, D.; Podoplelova, Y.; Piehler, J.; The stability of the ternary interferon-receptor complex rather than the affinity to the individual subunits dictates differential biological activities. J. Biol. Chem. 2008, 283, 32925-32936.
- Michaelis, V.; Menten, M. Die Kinetik der Invertinwirkung (The Kinetics of Invertase Action). Biochemische Zeitschrift 1913, 49, 33.
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems, Wiley-Interscience, 1993

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.