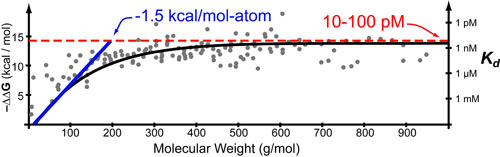
Not too long ago, some really cool papers1,3 sought to examine “The Maximal Affinity of Ligands” by compiling a list of known small-molecule drugs and comparing their affinities (in kcal/mol or Kd‘s see post on the Hill Equation) with various parameters such as molecular weight (see figure above).1
Interestingly, they found two approximately linear regimes: (1) a linear increase in Kd with molecular weight at low molecular weights and (2) a plateau at Kd ~ 10 pM at higher molecular weights. It turns out that both of these regimes can be explained by some basic intuitive theory where
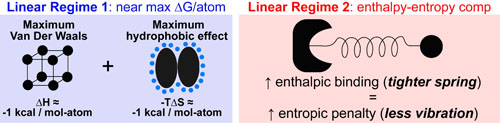
- The slope of regime 1 is approximately equal to: the sum of the maximum Van der Waals (enthalpy) and maximum hydrophobic effects (entropy) per heavy atom in a molecule.1,2
- Enthalpy/Entropy Compensaton explains the regime 2 plateau as once the hydrophobic effect (displaced water) has been maximized, entropy and enthalpy no longer work together but exactly oppose each other. A conceptual image of this effect is: tighter binding (improved enthalpy) was shown to directly results in an equivalent reduction in molecular vibration.3,4
REFERENCES:
- Kuntz, I. D.; Chen, K.; Sharp, K. A.; Kollman, P. A. The maximal affinity of ligands. PNAS, 1999, 96, 9997-10002.
- Dunitz, J. The entropic cost of bound water in crystals and biomolecules. Science, 1994, 264, 670.
- Gilli, P.; Ferretti, V.; Gilli, G.; Borea, P.; AEnthalpy-Entropy Compensation in Drug-Receptor Binding. J. Phys. Chem. 1994, 98, 1515-1518.
- Dunitz, J. Win some, lose some: enthalpy-entropy compensation in weak intermolecular interactions. Chemistry and Biology, 1995, 2, 709-712.

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.