Fluorescence (i.e. the emission of light from a electronically excited substance) is one of the most utilized physical phenomena in chemistry and biology. Though humans have been aware of fluorescence for thousands of years (e.g. fireflies), it wasn’t until the discovery of quinine in 1845 that we really started to understand its chemical basis(see figure above). Interestingly, during World War II, the study of quinine’s anti-malarial properties led to the development of the first spectrofluorometers which enabled true quantitative study of fluorescence. Finally, in the 1980-1990’s, new tools in synthetic chemistry and molecular biology allowed rational engineering of chemical fluorophores into a critical class of probes in biophysics(microscopy), molecular biology(sequencing), cell-biology(flow cytometry) and even anatomy (histology)).
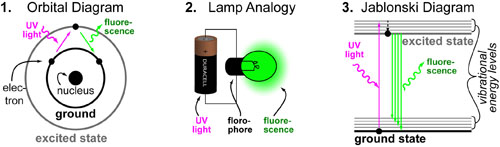
Above are three different pictures of how “fluorescence” works.
- The first Bohr atom “Orbital Diagram” shows how high energy ultraviolet (UV) light can “excite” an electron from its lowest energy orbital(ground state) to a higher energy orbital (excited state). Just like a high energy “lightening bolt throwing someone up into the sky”, this excited state is not stable and must “fall back to earth”. In fluorescence, the potential energy held in the excited state is released as visible light.
- It is often useful to think of fluorescence as a lamp where the high energy UV light can be thought of as a battery while the fluorophore is a lamp that converts that electronic energy into light.
- Finally, the Jabloski Diagram is one of the most commonly used diagrams for this process. Here, ground states and excited states are illustrated as horizontal lines (not single electron orbitals). The biggest difference between this illustration and the Orbital Diagram is in the inclusion of “vibrational energy levels.” These closely spaced sub-energies represent the different types of vibrations molecules have (during an excitation process) which can increase or decrease the spacing between excited and ground states.
REFERENCES:
- Lakowicz, J. R. in Principles of Fluorescence Spectroscopy, Springer, 2006
- McQuarrie, D. A.; Simon, J. D. Physical Chemistry: A Molecular Approach., University Science Books, 1999
- Skoog, D. A., Holler, F. J. & Nieman, T. A. Principles of Instrumental Analysis (5th ed), Saunders College Publishing, 1998