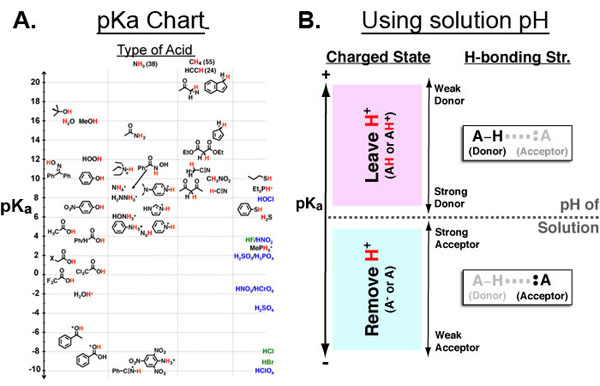
Figure 1 A. Chemical Functional Groups organized by pKa (y-axis) and acidic atom (x-axis: oxygen, nitrogen, carbon, other.) B. Key for using A given a solution pH. First, mark the position of the solution pH on the pKa axis (dotted horizontal line). all functional groups above it are neutral or positively charged while all functional groups below it are negative or neutral. Second, the pKa axis is useful in further categorizing functional groups by their ability to participate in hydrogen bonds.
The pKa value of a chemical functional group (Figure 1A) is very useful because it can directly give you the approximate charged state of that functional group (in the context of drugs, proteins, membranes, DNA, etc.) at a specific solution pH. As such, the pKa is critical to an intuitive understanding of electrostatics in chemical and biological contexts.
When the pKa of a functional group equals the solution pH, the ratio of protonated to deprotonated form of that functional group is 50/50 (i.e., HA/A– = HA+/A). Otherwise (Figure 1B):
- pKa > pH of solution → group is neutral or positive (HA or HA+)
- pKa < pH of solution → group is negative or neutral (A– or A)
This rule of thumb is quantified by the Henderson Hasselbalch equation:

and is very useful for explaining electrostatics in basic chemistry and biology (e.g. enzyme kinetics, charged state of proteins, drugs, etc.) experimental methods in biology and chemistry (e.g. electrophoretic mobility, chemical extraction) and medicine (e.g. oral biolavailability/membrane permiability of drugs)
In addition to giving information about the charged state of a small molecules and macromolecules the pKa can serve as a metric for the relative hydrogen bonding strength of different functional groups as donors (in the protonated state pH < pKa) or acceptors (in the deprotonated state pH > pKa) which is also improtant throughout the chemical and biological sciences (e.g. protein folding, chemical catalysis, etc.)
pKa Sheet PDF for mobile devices
This work was inspired by the Professor Evans’ pKa tables which can be found on the Evans Group Page at Harvard.

This work by Eugene Douglass and Chad Miller is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
