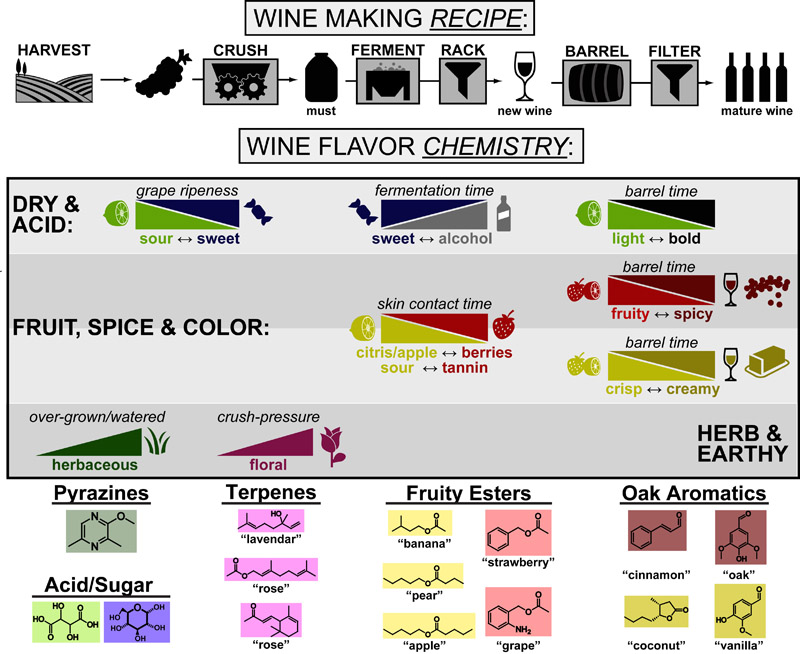
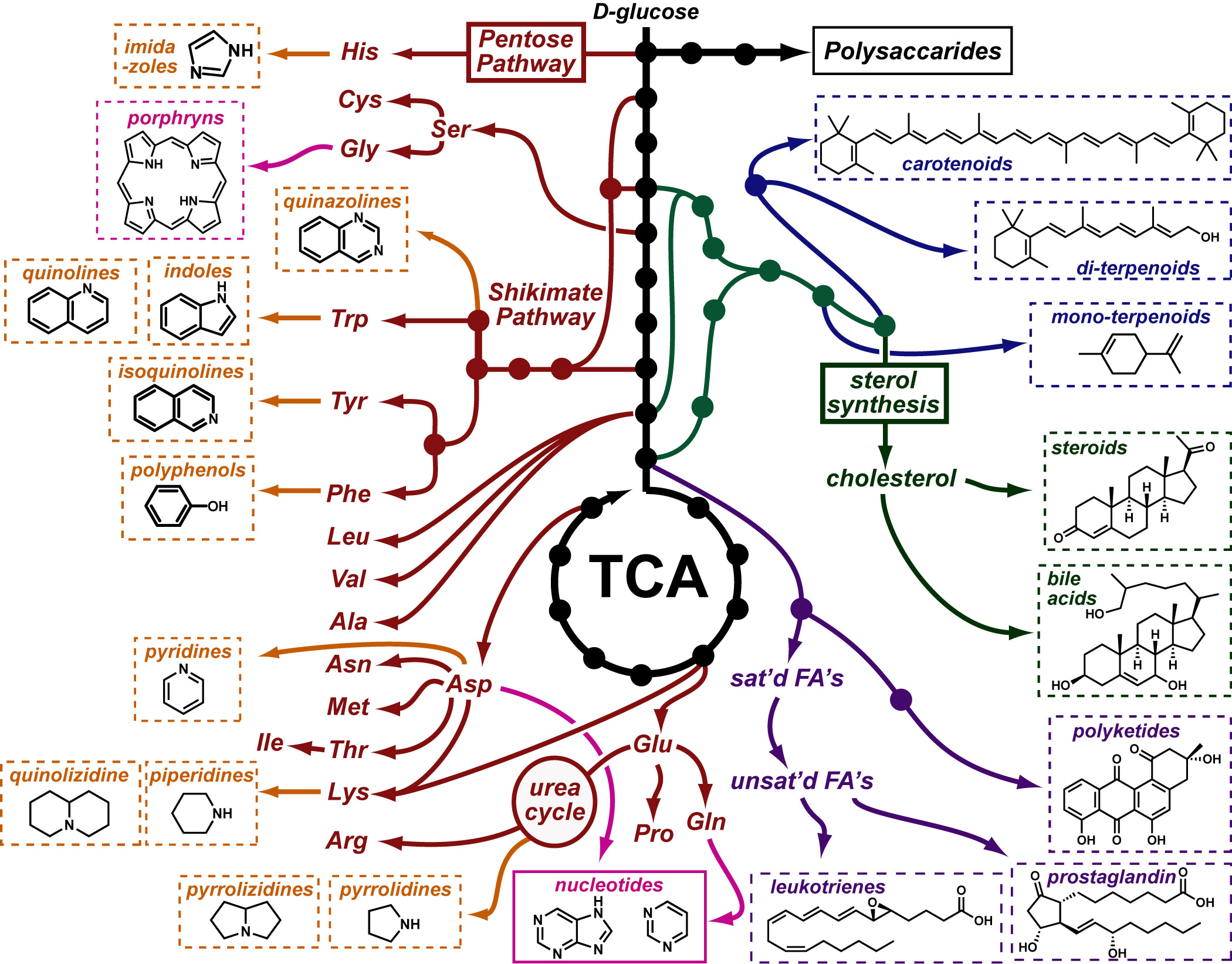
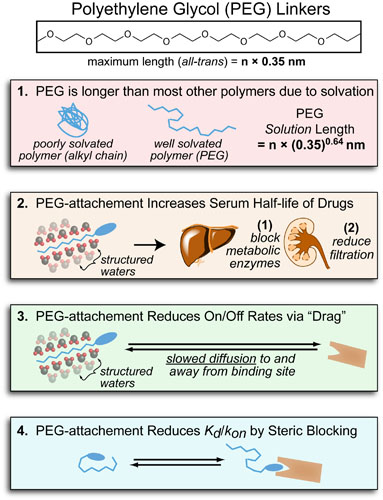
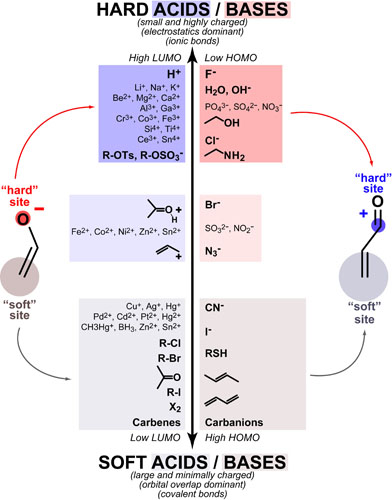
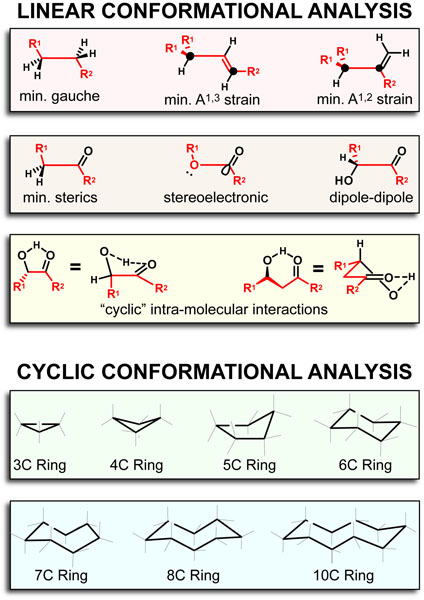
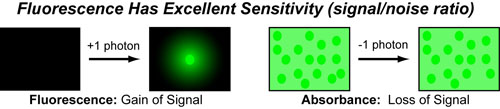
Our recent whiskey-graphic, led to some requests for a similar graphic on wine. Based on our research, wine appears to have a simpler “process chemistry” but more complicated “flavor chemistry” than whiskey. While we are still anchoring this graphic in the “production process chemistry” its major focus is on how individual steps affect the flavor of a wine.
About Us
Practically Science was started by two Yale PhD students in 2012. Its goal is to make single-sheet summaries of common interdisciplinary methods, ideas, etc. Continue Reading →Please follow & like us :)
Blogroll
Site Map
-
Recent Posts